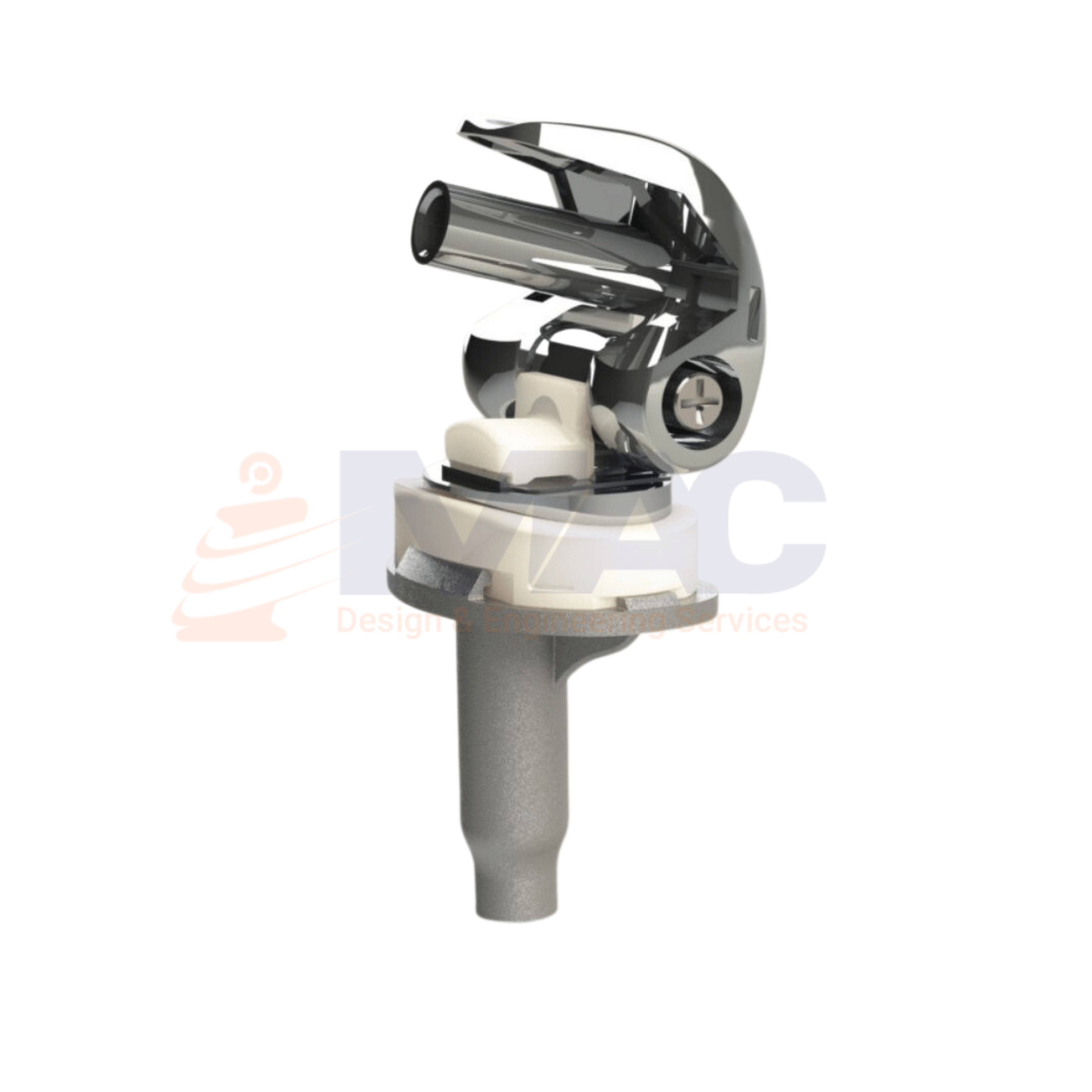
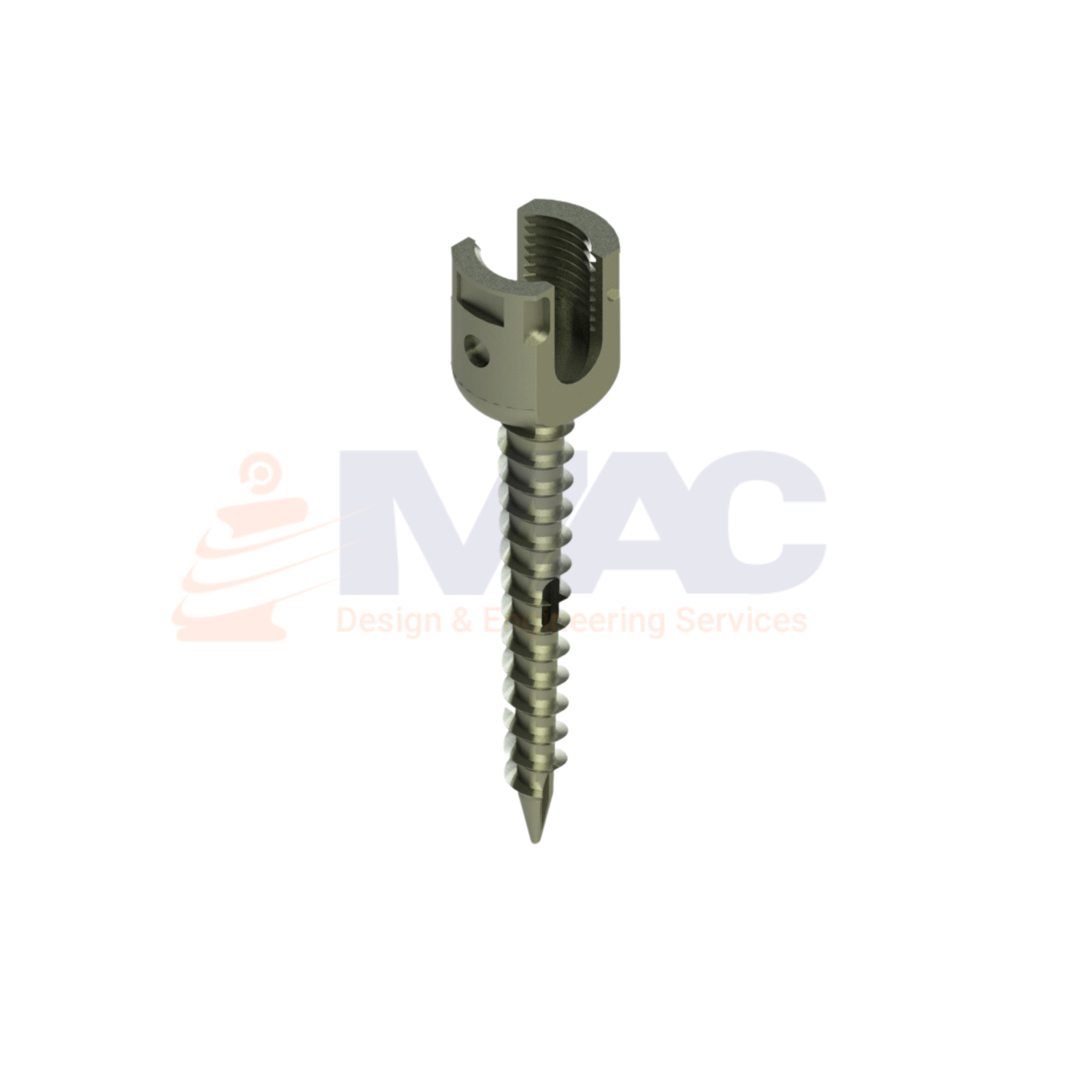
Medical equipment design requires pushing boundaries of technology and leveraging communication tools to create highly reliable, portable, and user-friendly medical equipment.
Our Capabilities to develop medical devices in this segments as IntraUterine Devices, Biopsy CT Scan Assistive Devices, Ultrasonic Piezoelectric Dental Scaler, X Ray Machine, ECG recorder design, Cardiac Stress Test TreadMill design and ICU multiparameter monitor.
We do reverse Engineering of the Medical Surgical Equipments & tools in such a way to make it complete new modified design and adaptive. Our design engineer team focuses on human centered medical device design & development.